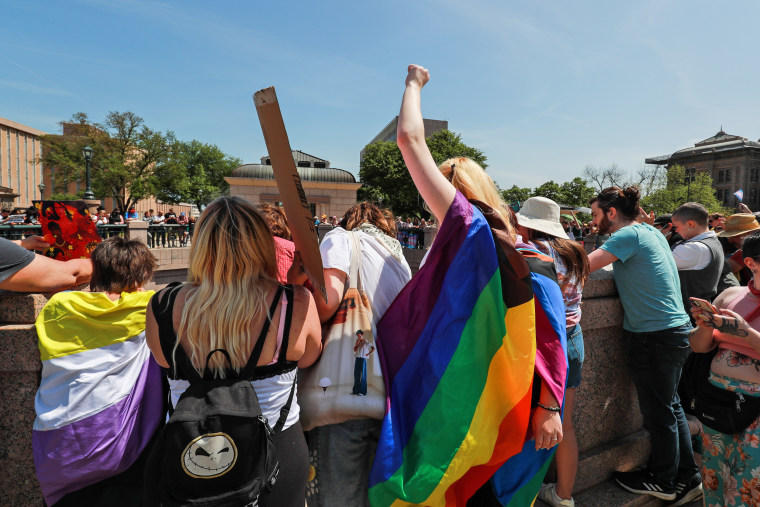Abortion Drug Mifepristone Still Available — With Limitations
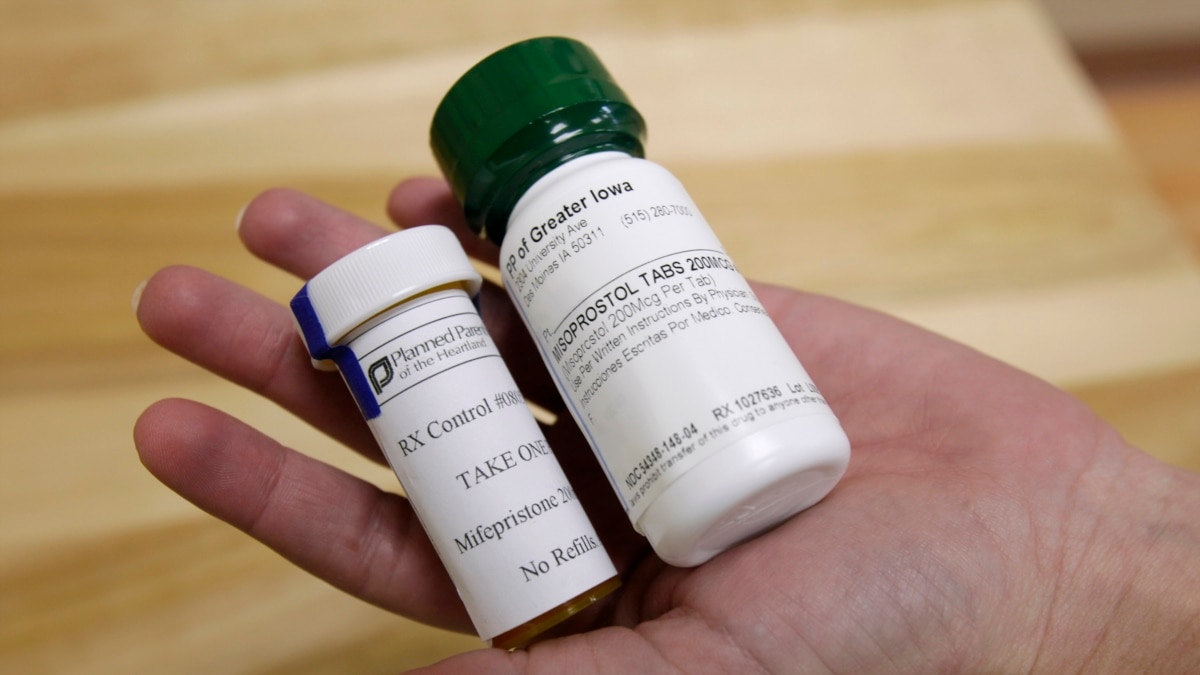
The U.S. Department of Justice said Thursday it would seek an emergency order from the Supreme Court to suspend the restrictions in response to a ruling restricting access to the abortion drug mifepristone.
After various courts handed down conflicting rulings on mifepristone, the abortion-inducing pill and the most commonly used method in the United States, a federal appeals court on Wednesday said it could be used for now. has ruled that there are some restrictions.
Mifepristone has been approved by the Food and Drug Administration for 23 years. Last week, Texas U.S. District Court Judge Matthew Kaksmalik revoked approval of the pill’s use following a lawsuit by anti-abortion advocates.
Less than an hour later, a Washington judge ordered the FDA to maintain access to the drug in 17 states and the District of Columbia.
In response, the New Orleans-based Court of Appeals for the Fifth Circuit voted late Wednesday to temporarily narrow Kacsmaryk’s ruling.
In a two-to-one vote, a judge in the Court of Appeals suspended changes in 2016 and 2021 that would have eased the FDA’s rules for prescribing and dispensing mifepristone. Relaxed rules include allowing pills to be mailed, lifting the requirement for three in-person doctor visits, and authorizing use of the drug up to 10 weeks instead of 7. I was.
By preventing pills from being mailed, access to abortion is reduced. The reversal of Roe v. Wade less than a year ago has resulted in more than 10 states outright banning abortion. Roe vs. Wade was a case that recognized the constitutional right to abortion.
reaction to the verdict
In a statement, Attorney General Merrick Garland said, “We intend to seek urgent relief from the Supreme Court to defend the FDA’s scientific judgment and protect Americans’ access to safe and effective reproductive care. Unless an emergency order is issued, the appeals court’s ruling will take effect on Saturday.
Vice President Kamala Harris also issued a statement.
“There is a reproductive health care crisis in America. Our administration will continue to fight to defend women’s health and their right to make decisions about their own bodies,” she wrote.
Erin Hawley, lead attorney for the plaintiffs in the case, expressed satisfaction with the latest verdict.
“The Fifth Circuit’s decision is an important victory for the doctors we represent, for women’s health, and for all Americans who deserve an accountable federal government that acts within the bounds of the law,” said the Conservative. said Hawley, a senior attorney at the leading legal advocacy group Alliance Defending Freedom. .
Supporters or opponents of abortion rights can file a lawsuit in the Supreme Court. Drug opponents may seek to keep the Court of Appeal’s full judgment in force. Alternatively, the Biden administration could ask the Supreme Court to allow all FDA changes to remain in place while the case moves through the legal system.
Judgment Alleges Inadequate Examination
At the heart of the Texas ruling is the allegation that the FDA’s initial approval of mifepristone was flawed.
Common side effects include cramping, bleeding, nausea, headache, and diarrhea. In rare cases, women may experience excessive bleeding that requires surgical intervention.
As of June 2022, more than 5.6 million women in the United States were using the drug, according to the FDA. During that period, the agency received reports of her 4,200 complications, which was less than her 1/10th of her 1% of women taking the drug.
In easing restrictions on mifepristone, FDA regulators cited a “very low rate of serious adverse events.”
More than 250 pharmaceutical executives criticized the Texas judge’s decision in an open letter. They said they ignored decades of scientific evidence and legal precedent.
In the letter they wrote: ”
Some information in this report was provided by The Associated Press, Agence France Presse and Reuters.
https://www.voanews.com/a/abortion-drug-mifepristone-to-remain-available-with-restrictions/7049653.html Abortion Drug Mifepristone Still Available — With Limitations